|
Tariq Shafi, MD, MHS-Nephrologist
Acting Professor of Medicine, Academic Institute Head, Division of Kidney Diseases, Hypertension & Transplantation, Department of Medicine Houston Methodist
Dr. Shafi is an NIH-funded clinician-scientist focusing on cardiovascular risk factors and outcomes in patients with kidney disease treated with dialysis. Dr. Shafi has extensive expertise in human subjects clinical research, biomarker studies, health services research, and population health. He has been continuously funded by NIH since 2006. Dr. Shafi is a permanent member of the NIH Health Services Quality and Effectiveness (HSQE) Study Section and is a member of the editorial boards of the Clinical Journal of the American Society of Nephrology, the American Journal of Medical Sciences, and Kidney360.
|

|
Finnian McCausland, MBBCh, MMSc, FRCPI, PG CertMedEd- Nephrologist
Dr. Finnian R. Mc Causland is an assistant professor of medicine, faculty director in Postgraduate Medical Education and co-director of the Master of Medical Sciences in Clinical Investigation program at Harvard Medical School. He is also an associate physician and attending nephrologist at Brigham and Women's Hospital (BWH). Dr. McCausland's major research interests relate to the cardiovascular consequences associated with hemodialysis and risk factors for progression of chronic kidney disease. He graduated from Harvard Medical School with a Master’s in Medical Science Degree in Clinical Investigation in 2012 and has received competitive funding from the Irish Nephrology Society, American Heart Association, National Kidney Foundation and National Institutes of Health. He is the principal investigator of two ongoing randomized controlled trials related to the dialysate prescription and its effect on hemodynamic stability.
|

|
Daniel E. Weiner, MD, MS-Nephrologist
Associate Medical Director, Dialysis Clinic, Inc., Boston; Associate Professor, Tufts University School of Medicine
Dr. Weiner is the recipient of an award from PCORI to evaluate decision-making in advanced kidney diseases. He is the prior recipient of an R01 from the NIDDK investigating the role of exercise on vascular disease, physical function and cognitive function in people with chronic kidney disease. He previously completed a K23 Career Development Award from the NIDDK investigating cerebrovascular disease and cognitive impairment in individuals with CKD. He is the local principal investigator in multiple clinical trials evaluating people with chronic kidney disease, including the NIH-funded SPRINT trial, investigating optimal blood pressure targets in older adults with cardiovascular disease or CKD.
Dr. Weiner also conduct research in the dialysis population, examining dialysis practices, and he is the national principal investigator for a large pragmatic clinical trial evaluating nutritional supplements in hemodialysis patients. Other ongoing research evaluates cognitive impairment and cerebrovascular disease in dialysis patients. He also is collaborating with researchers at Boston University and in Nicaragua to investigate causes of epidemic CKD in Central America.
|

|
Kaleab Z. Abebe, PhD- Biostatistician
• Assistant Vice Chancellor for Clinical Trials, Health Sciences
• Professor of Medicine with Tenure
• Associate Professor of Biostatistics and Clinical and Translational Science
• Director, Center for Research on Health Care Data Center (CRHC-DC)
• Director, Center for Clinical Trials and Data Coordination (CCDC)
Dr. Abebe's collaborative research focuses on design, conduct, coordination, and analysis of multicenter randomized controlled trials (RCTs). He is the PI of two large NIH-funded consortiums: the COPE-AKI Consortium Scientific & Data Research Center, which is developing and testing interventions to reduce morbidity and mortality in AKI survivors; and the Data Coordinating Center (DCC) for the REBIRTH Study, which will assess the effect of bromocriptine on left ventricular ejection fraction in women with peripartum cardiomyopathy. He is the Co-PI for the CaRISMA study, which is a pragmatic trial examining the effectiveness of computerized cognitive behavioral therapy (vs pain education) on pain intensity in adults with sickle cell disease (SCD). Dr. Abebe’s methodological interests revolve around the design and analysis of cluster randomized trials. In addition to his research collaborations, Dr. Abebe is the director of the Clinical Trials Track for the MS in Clinical Research at the Institute for Clinical Research Education. He is a standing member of the Kidney, Endocrine, and Digestive Disorders (KEDD) Study Section, and he is a member of the Board of Directors and chair of the Equity, Diversity, and Inclusion committee for the Society for Clinical Trials.
|

|
Paul T. Conway
Mr. Conway is Chair of Policy & Global Affairs, and past president of the American Association of Kidney Patients (AAKP), the largest U.S. kidney patient organization. Mr. Conway has advanced the principles of patient consumer care choice, patient-centered care, and greater use of patient insight data in research and innovation across the federal government and, globally, before the United Nations, the European Parliament, and the World Health Organization.
|

|
Fiona McKinney-Patient Ambassador
Fiona is an active AAKP Ambassador who got involved as a kidney advocate to ensure better health coverage for dialysis and transplant patients, including coverage for kidney transplant medications (immunosuppressants) and post-transplant care.
|
| |
Yalonda Moore-Caregiver Ambassador
In February 2018, Yalonda was asked to join the NPFE-LAN Team. She has been a member as a SME for Caregivers/Care Partners. Both Gary and Yalonda have participated in several podcasts as well as educational review panels. They love talking about the benefits of doing PD and traveling while on PD as well as talking to people about how to involve your children in your treatment and being your own first defense during your treatment.
|
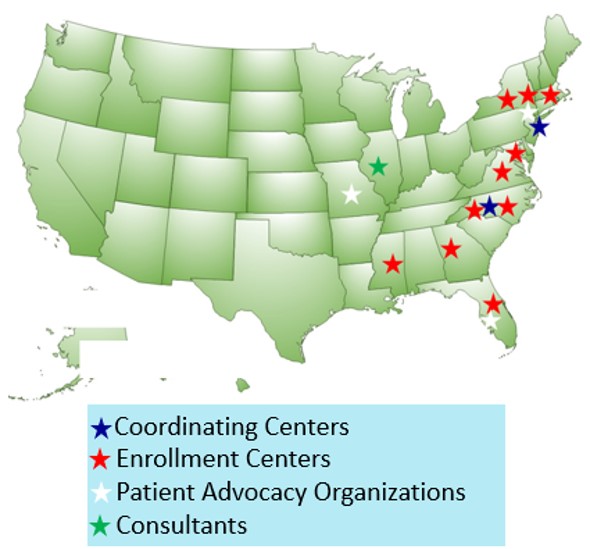
 The TwoPlus Clinical Trial is funded by Patient-Centered Outcomes Research Institute (PCORI). The study takes place at ten healthcare systems and more than fifty outpatient dialysis units in the United States. The study is conducted in collaboration with patient-led organizations like the American Association of Kidney Patients and Home Dialyzors United.
The TwoPlus Clinical Trial is funded by Patient-Centered Outcomes Research Institute (PCORI). The study takes place at ten healthcare systems and more than fifty outpatient dialysis units in the United States. The study is conducted in collaboration with patient-led organizations like the American Association of Kidney Patients and Home Dialyzors United.